A Planar Nickelaspiropentane Complex with Magnesium-Based Metalloligands: Synthesis, Structure, and Synergistic Dihydrogen Activation
Yanping Cai1, Shengjie Jiang1, Thayalan Rajeshkumar2, Laurent Maron2,*, and Xin Xu1,*(徐信)
1 Key Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou 215123, P. R. China
2 LPCNO, CNRS & INSA, Université Paul Sabatier, 31077 Toulouse, France
J. Am. Chem. Soc. 2022, 144, 16647--16655
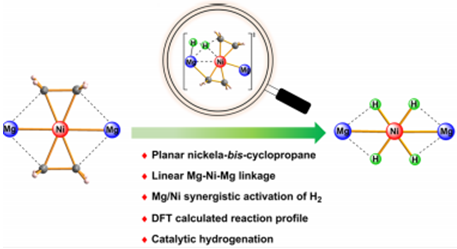
The nature of transition-metal–olefin bonding has been explained by the Dewar–Chatt–Duncanson model within a continuum of two extremes, namely, a π-complex and a metallacyclopropane. The textbook rule suggests that a low-spin late-transition-metal–ethylene complex more likely forms a π-complex rather than a metallacyclopropane. Herein, we report a low-spin late-transition-metal–bis-ethylene complex forming an unprecedented planar metalla-bis-cyclopropane structure with magnesium-based metalloligands. Treatment of LMgEt (L = [(DippNCMe)2CH]−, Dipp = 2,6-iPr2C6H3) with Ni(cod)2 (cod = 1,5-cyclooctadiene) formed the heterotrimetallic complex (LMg)2Ni(C2H4)2, which features a linear Mg–Ni–Mg linkage and a planar coordination geometry at the nickel center. Both structural features and computational studies strongly supported the Ni(C2H4)2 moiety as a nickelaspiropentane. The exposure of (LMg)2Ni(C2H4)2 to 1 bar H2 at room temperature produced a four-hydride-bridged complex (LMg)2Ni(μ-H)4. The profile of H2 activation was elucidated by density functional theory calculations, which indicated a novel Mg/Ni cooperative activation mechanism with no oxidation occurring at the metal center, differing from the prevailing mono-metal-based redox mechanism. Moreover, the heterotrimetallic complex (LMg)2Ni(C2H4)2 catalyzed the hydrogenation of a wide range of unsaturated substrates under mild conditions.
链接:https://pubs.acs.org/doi/10.1021/jacs.2c07402