报告题目:Anionic Polymerization of Monomers Possessing Adamantyl Groups
报告人:Prof. Takashi Ishizone,Tokyo Institute of Technology
时间:2018年9月25日(星期二)下午14:00-15:00
地点:苏大独墅湖校区911楼401室

CV:
Education and Professional Experience:
2016-present: Professor, Department of Chemical Science and Engineering, Tokyo Institute of Technology
2014-2015: Professor, Department of Organic and Polymeric Materials, Tokyo Institute of Technology
2000-2014: Associate Professor, Department of Organic and Polymeric Materials, Tokyo Institute of Technology
1995-1996: Visiting Scholar, Department of Chemistry, The University of Chicago (P. E. Eaton), USA
1994: Ph. D. from Graduate School of Science and Engineering, Tokyo Institute of Technology
1989-2000: Assistant Professor, Department of Polymer Chemistry, Tokyo Institute of Technology
1988: M. S. from Graduate School of Science and Engineering, Tokyo Institute of Technology
1986: B. S. from Faculty of Engineering, Tokyo Institute of Technology, Tokyo, Japan
Research Interests:
Thus far, Prof. Ishizone has published more than 140 research papers and review articles in peer-reviewed journals such as Prog. Polym. Sci., JACS, Adv. Mater., Chem. Commun., ACS Macro Lett. and Macromolecules, about 17 books and book chapters, and been authorized more than 20 patents. His research interests primarily include living anionic polymerization, organic chemistry, synthesis of functional polymer, polymers possessing adamantyl groups, and water-soluble thermo-responsive polymer.
Representative Publications:
1) Synthesis of Polymers Carrying Adamantyl Substituents in Side Chain, T. Ishizone, R. Goseki, Polym. J. 2018, 50, 805.
2) Living Anionic Polymerization of 4-(1-Adamantyl)-α-methylstyrene,S. Kobayashi, H. Kataoka, R. Goseki, T. Ishizone, Macromol. Chem. Phys. 2018, 219, 1700450.
3) A Segregation and Deprotection Approach for Hydrophilic Surfaces Using Amphiphilic Block Copolymers Possessing Polystyrene and Poly[(tri(ethylene glycol) methacrylate)] Segments,A. Seki, T. Ishizone, A. Oyane, H. Yokoyama, Macromol. Chem. Phys.2017, 218, 1700048.
4) Living Anionic Polymerization of 1-Adamantyl 4-vinylphenyl ketone, D. Matsuoka, R. Goseki, S. Uchida, T. Ishizone, Macromol. Chem. Phys. 2017, 218, 1700015.
5) Synthesis and surface characterization of well-defined amphiphilic block copolymers composed of polydimethylsiloxane and poly[oligo(ethylene glycol) methacrylate], R. Goseki, L. Hong, M. Inutsuka, H. Yokoyama, T. Ishizone,RSC Adv.2017, 7, 25199.
6) Living Anionic Polymerization of N‑(1-Adamantyl)‑N‑4-vinylbenzylideneamine and N‑(2- Adamantyl)‑N‑4-vinylbenzylideneamine: Effects of Adamantyl Groups on Polymerization Behaviors and Thermal Properties, B.-G. Kang, H. Shoji, H. Kataoka, R. Kurashima, J.-S. Lee, T. Ishizone, Macromolecules2015, 48, 8489.
7) Living anionic polymerization ofα-methyleneindane: An exo-methylene hydrocarbon monomer, H. Ohishi, Y. Kosaka, K. Kitazawa, R. Goseki, S. Kawauchi, T. Ishizone, Macromolecules2015, 48, 6900.
8) Formation of Alternating Copolymers via Spontaneous Copolymerization of 1,3-Dehydroadamantane with Electron-Deficient Vinyl Monomers, S. Matsuoka, N. Ogiwara, T. Ishizone, J. Am. Chem. Soc. 2006, 128, 8708.
Abstract:
It has been shown that incorporation of bulky and stiff adamantane skeleton into the backbone or as a pendant group into the polymers induces drastic changes in the physical and chemical properties. We succeeded in the living anionic polymerization of a series of styrene derivatives carrying adamantyl groups,1-7, to afford the well-defined polystyrenes exhibiting markedly high Tg values.1-5) For example, the usual anionic polymerization of 12) quantitatively occurred with sec-BuLi in THF at –78 °C, while an a-methylstyrene derivative of 2 showed a typical equilibrium polymerizability under the anionic condition.3) Anionic polymerization of new isomeric p-divinylbenzene (pDVB) derivativescontaining adamantyl groups at one of the vinyl groups of pDVB framework, 3 and 4, was also carried out with sec-BuLi in THF at –78 °C. The polymerization of 3 and 4 exclusively proceeded at the styryl frameworks within 1 h to form the polymers with predicted molecular weights and narrow molecular weight distributions (Mw/Mn = 1.1). Although the anionic polymerizability of hydrocarbon monomers, 1-4, was very similar to styrene or a-methylstyrene, 54) and 65) showed significantly higher anionic polymerizabilities because of the electron-withdrawing effects of the C=O and CH=N-R substituents. A bulky p-conjugated carbanion, such as diphenylmethylpotassium, with low nucleophilicity can be used as an effective anionic initiator for 5 and 6 to form the well-defined polymers. In the case of 7, the N=C substituent acted as an electron-donating group in the polymerization. The acidic hydrolyses of poly(6)5) and poly(7) quantitatively proceeded to afford a poly(4-formylstyrene) and a poly(4-aminostyrene), respectively. The resulting polymers with adamantyl groups started to decompose over 320 °C and showed remarkably high Tgs compared with the polystyrene (Tg = 100 °C). In fact, the Tg values of poly(1), poly(2), poly(3), poly(4), poly(5), poly(6), and poly(7) were observed at 234, 274, 253, 173, 193, 257, and 169 °C, indicating the effects of adamantyl substituents and the linkage between the adamantyl groups and the polystyrene main chain. The anionic polymerizations of 2-(1-adamantyl)-5-vinylthiophene, 8, 2-(1-adamantyl)-1,3-butadiene, 9,1) and 3-methacryloyloxy-1,1’-biadamantane, 10,1) quantitatively proceeded in the living fashion to give the polymers of tailored chain structures. The Tg values of poly(8), poly(9), and poly(10) were 198, 106, and 236 °C, again supporting the drastic effect of adamantyl group to increase the Tg values.

References
1) T. Ishizone, R. Goseki, Polym. J. 2018, 50, 805.
2) S. Kobayashi, T. Matsuzawa, S. Matsuoka, H. Tajima, T. Ishizone, Macromolecules2006, 39, 5979.
3) S. Kobayashi, H. Kataoka, R. Goseki, T. Ishizone, Macromol. Chem. Phys. 2018, 219, 1700450.
4) D. Matsuoka, R. Goseki, S. Uchida, T. Ishizone, Macromol. Chem. Phys. 2017, 218, 1700015.
5) B.-G. Kang, H. Shoji, H. Kataoka, R. Kurashima, J.-S. Lee, T. Ishizone, Macromolecules2015, 48, 8489.
(二)
报告题目:Designed High-chi Block Copolymers Toward Directed Self-Assembly (DSA) Lithography
报告人:Prof. Teruaki Hayakawa,Tokyo Institute of Technology
时间:2018年9月25日(星期二)下午15:00-16:00
地点:苏大独墅湖校区911楼401室

CV:
Teruaki Hayakawa received his B.S. (1995), M.S. (1997) and Ph.D. (2000) from Yamagata University with Prof. Mitsuru Ueda for research in the fields of materials science and engineering. During 1997-1998 he was a visiting student with Prof. Christopher K. Ober at Cornell University. In 2000 he joined the National Institute of Advanced Industrial Science and Technology (AIST) as a researcher and moved to Tokyo Institute of Technology in 2003. He is currently a Professor in the Department of Materials Science and Engineering, School of Materials and Chemical Technology, Tokyo Institute of Technology. He was a chairperson of the research group on Polymers for Microelectronics and Photonics, the Society of Polymer Science, Japan (SPSJ) in 2012-2014. He is currently an Executive Director of the SPSJ in 2018-2020. He has received the Award of Encouragement of Research in Polymer Science National Science, SPSJ 2005, SPSJ Award for the Outstanding Paper in Polymer Journal 2006 sponsored by ZEON, SPSJ Hitachi Chemical Award 2010, SPSJ Wiley Award 2015, and The Photopolymer Science and Technology Award, The Best Paper Award 2016. Thus far, he has published more than 120 research papers and review articles in journals such as JACS, Adv. Mater., Adv. Funct. Mater., Angew. Chem., ACS Macro Lett. and Macromolecules, about 8 books and book chapters, and been authorized more than 40 patents.
Research Interests: organic and polymer synthetic chemistry, especially for living anionic polymerization and polycondensation, nanomaterials science and fabrication based on condensation aromatic polymers, self-assembly and directed self-assembly of block copolymers, and high thermal conductive liquid crystalline epoxy adhesives.
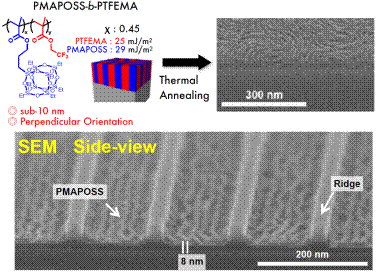
Abstract: Block copolymers (BCPs) which are able to spontaneously self-assemble into discrete nanostructures have been proposed as a promising candidate for next generation nanopatterning technologies, namely directed self-assembly (DSA), enabling high-resolution patterning in thin films over large areas at low cost. Silicon-containing BCPs have garnered significant attention for achieving sub-10 nm feature sizes required for next generation lithography techniques. In addition, the high etching contrast between the hydrocarbon polymer block and the silicon-containing polymer block allows for facile pattern transfers to substrates upon oxygen plasma etching. However, the hydrophobicity and lower surface energy of the silicon-containing polymer block prevents the lamellar structure from forming with a perpendicular orientation that is vital for line patterning lithography.
Herein, a series of perpendicular lamellae-forming high-chi block copolymers are described that have been developed based on the bottom-up concept of a simple yet effective material that adjusts the chemical properties and molecular composition of the material. One example is newly designed and successfully synthesized silicon- and fluorine-containing high-chi BCP, poly(polyhedral oligomeric silsesquioxane-block-2,2,2-trifluoroethyl methacrylate) (PMAPOSS- b-PTFEMA) whose surface free energies of the constituents are perfectly balanced. The perpendicularly oriented BCP thin films were fabricated using simple spin-coating and thermal annealing processes under ambient conditions. The thin films displayed a minimum domain size of L0 = 11 nm. The formation of microphase-separated nanostructures was observed in in-situ Atomic Force Microscopy.
Selected publications:
1) Self-Assembly of Hierarchical Structures Using Cyclotriphosphazene-Containing Poly(substituted methylene) Block Copolymers, F. Kato, A. Chandra, M. Tokita, H. Asano, H. Shimomoto, E. Ihara, T. Hayakawa, ACS Macro Lett.2018, 7, 37.
2) Perpendicular Orientation Control without Interfacial Treatment of RAFT-Synthesized High‑χ Block Copolymer Thin Films with Sub-10 nm Features Prepared via Thermal Annealing, R. Nakatani, H. Takano, A. Chandra, Y. Yoshimura, L. Wang, Y. Suzuki, Y. Tanaka, R. Maeda, N. Kihara, S. Minegishi, K. Miyagi, Y. Kasahara, H. Sato, Y. Seino, T. Azuma, H. Yokoyama, C. K. Ober, T. Hayakawa, ACS Appl. Mater. Interfaces2017, 9, 31266.
3) Perpendicularly oriented sub-10-nm block copolymer lamellae by atmospheric thermal annealing for one minute, T. Seshimo, R. Maeda, R. Odashima, Y. Takenaka, D. Kawana, K. Ohmori, T. Hayakawa, Sci. Rep.2016, 6, 19481.
4) Hydrogen Bond Interactions Mediate Hierarchical Self-Assembly of POSS-Containing Block Copolymers Blended with Phenolic Resin, C.-W. Chiou, Y.-C. Lin, L. Wang, R. Maeda, T. Hayakawa, S.-W. Kuo, Macromolecules2014, 47, 8709.
5) Cylindrical Nanostructure of Rigid-Rod POSS-Containing Polymethacrylate from a Star-Branched Block Copolymer, R. Goseki, A. Hirao, M. Kakimoto, T. Hayakawa, ACS Macro Lett. 2013, 2, 625.
6) Hierarchical Structure in Nanoscale Thin Films of a Poly(styrene-b-methacrylate grafted with POSS) (PS214-b-PMAPOSS27), B. Ahn, T. Hirai, S. Jin, Y. Rho, K.-W. Kim, M. Kakimoto, P. Gopalan, T. Hayakawa, M. Ree, Macromolecules2010, 43, 10568.
7) Hierarchical Self-Assembled Structures from POSS-Containing Block Copolymers Synthesized by Living Anionic Polymerization, T. Hirai, M. Leolukman, S. Jin, R. Goseki, Y. Ishida, M. Kakimoto, T. Hayakawa, M. Ree, P. Gopalan, Macromolecules2009, 42, 8835.
8) One-Step Direct-Patterning Template Utilizing Self-Assembly of POSS-Containing Block Copolymers, T. Hirai, M. Leolukman, C. C. Liu, E. Han, Y. J. Kim, Y. Ishida, T. Hayakawa, M. Kakimoto, P. F. Nealey, P. Gopalan, Adv. Mater. 2009, 21, 4334.
9) Hierarchical Nanostructures of Organosilicate Nanosheets within Self-Organized Block Copolymer Films, T. Hirai, M. Leolukman, T. Hayakawa, M. Kakimoto, P. Gopalan, Macromolecules2008, 41, 4558.
欢迎感兴趣的老师和同学参加。
(报告联系人:赵优良老师)