Visible light-promoted ring-opening functionalization of unstrained cycloalkanols via inert C–C bond scission
Dongping Wang a, Jincheng Mao c and Chen Zhu *ab(朱晨)
a Key Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, 199 Ren-Ai Road, Suzhou, Jiangsu 215123, China.
b Key Laboratory of Synthetic Chemistry of Natural Substances, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai 200032, China .
c State Key Laboratory of Oil and Gas Reservoir Geology and Exploitation, Southwest Petroleum University, Chengdu 610500, China .
Chem. Sci., 2018, 9, 5805--5809
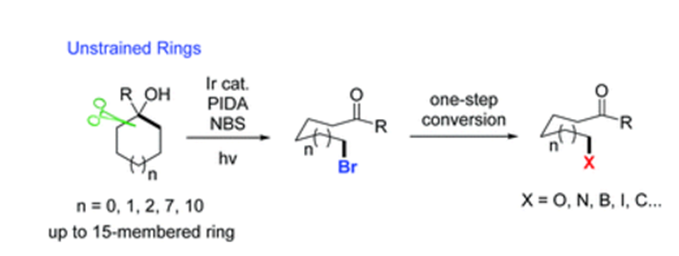
Described herein is a novel, useful, visible light-promoted ring-opening functionalization of unstrained cycloalkanols. Upon scission of an inert cyclic C–C σ-bond, a set of medium- and large-sized rings are readily brominated under mild reaction conditions to afford the corresponding distal bromo-substituted alkyl ketones that are hard to synthesize otherwise. The products are versatile building blocks, which are easily converted to other valuable molecules in one-step operation. This protocol is also applicable to the unprecedented ring-opening cyanation and alkynylation of unstrained cycloalkanols.
链接:http://pubs.rsc.org/en/Content/ArticleLanding/2018/SC/C8SC01763H#!divAbstract