Exogenous vitamin C boosts the antitumor efficacy of paclitaxel containing reduction-sensitive shell-sheddable micelles in vivo
Yaqin Zhua, b, Xiuxiu Wanga, Jian Zhanga, Fenghua Menga, Chao Denga, Ru Chenga, Jan Feijena, b,*, Zhiyuan Zhonga, *(钟志远)
aBiomedical Polymers Laboratory, and Jiangsu Key Laboratory of Advanced Functional Polymer Design and Application, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou 215123, PR China
bDepartment of Polymer Chemistry and Biomaterials, Faculty of Science and Technology, MIRA Institute for Biomedical Technology and Technical Medicine, University of Twente, P.O. Box 217, 7500 AE Enschede, The Netherlands
Journal of Controlled Release 250 (2017) 9–19
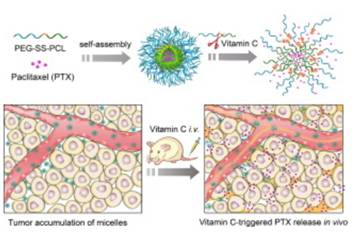
Slow drug release at the tumor tissue and poor tumor penetration are two big challenges for the successful application of nanosystems in tumor therapy. Here, we report that a high concentration of the natural reducing agent vitamin C (VC) triggers rapid extracellular PTX release from PTX-loaded shell-sheddable PEG-SS-PCL micelles (SSM) in tumors in vivo. An in vivo tolerance study showed that VC at a blood concentration of 40 mM had little toxicity to nude mice. Notably, SSM rapidly disassembled and released the payloads (Cy5 or PTX) in response to 40 mM VC. In vivo near-infrared imaging of tumor-bearing mice showed that with post-injection of VC to establish a blood concentration of 40 mM, Cy5 was quickly released from the micelles and diffused deep into the tumor tissue. Biodistribution studies revealed that 6 h after the injection of PTX-loaded micelles the highest tumor accumulation was reached, which was set as the injection time for VC. The antitumor efficacy of a combination therapy of PTX-loaded micelles and VC was evaluated in both MCF-7 and U87MG tumor models. In both tumor models, single injections of VC didn't show any antitumor effect, while sequential administration of PTX-loaded SSM and VC exhibited significantly higher tumor inhibition effects and better survival rates as compared to single treatment with PTX-loaded micelles, demonstrating that exogenous administration of VC effectively triggered the release of PTX from SSM in vivo. The combination of reduction-sensitive nanomedicines with exogenous VC appears a promising approach to achieve potent treatment of malignant tumors.
链接:http://www.sciencedirect.com/science/article/pii/S0168365917300573