Pincer-cobalt boosts divergent alkene carbonylation under tandem electro-thermo-catalysis
Shulei Ge1,2, Zhili Cui1,2, Lei Peng3,4, Xintong Wang5, Kaixin Chen1,2, Changrui Nie1,2, Shoucheng Dong1,2, Yang Huang3,4, Gen Luo(罗根)5*, Lin He(何林)3,4*, Jie Li1,2,6,7(李杰)*
1State Key Laboratory of Bioinspired Interfacial Materials Science, Soochow University, Suzhou, China.
2College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou, China.
3State Key Laboratory of Low Carbon Catalysis and Carbon Dioxide Utilization, Lanzhou Institute of Chemical Physics (LICP), Chinese Academy of Sciences, Lanzhou, China.
4State Key Laboratory for Oxo Synthesis and Selective Oxidation, Lanzhou Institute of Chemical Physics (LICP), Chinese Academy of Sciences, Lanzhou, China.
5Institutes of Physical Science and Information Technology, Anhui University, Hefei, China.
6Suzhou Key Laboratory of Pathogen Bioscience and Anti-infective Medicine, Soochow University, Suzhou, China.
7MOE Key Laboratory of Geriatric Diseases and Immunology, Soochow University, Suzhou, China.
Nat. Commun., 2025, 16, 8803.
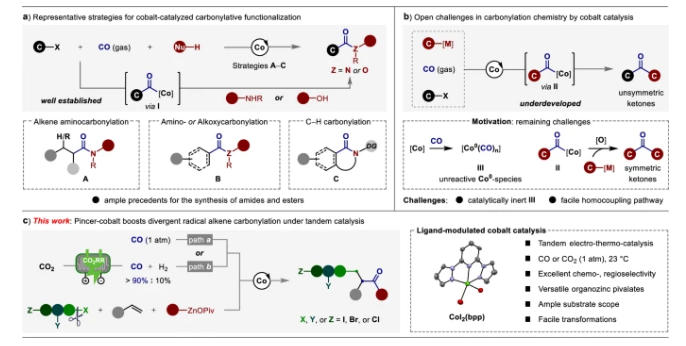
Abstract: Catalytic multicomponent carbonylation reactions with high regio- and chemoselectivity represent one of the long-pursued goals in C1 chemistry. We herein disclose a practical cobalt-catalyzed divergent radical alkene carbonylative functionalization under 1 atm of CO at 23 °C. The leverage of the tridentate NNN-type pincer ligand is the key to avoid the formation of catalytically inert Co0(CO)n species and overcome the occurrence of oxidative carbonylation of organozincs, selectively tuning the catalytic reactivity of cobalt center for dictating a full cobalt-catalyzed four-component carbonylation. Moreover, direct use CO2 as the C1 source in the multicomponent alkene carbonylative couplings can be achieved under a tandem electro-thermo-catalysis, thus allowing us to rapidly and reliably construct unsymmetric ketones with ample scope and excellent functional group compatibility. Remarkably, our protocol encompasses a broader of polyhaloalkanes as the electrophiles, which underwent radical-relay couplings in a completely regio- and chemoselective fashion. Finally, facile modifications of drug-like molecules demonstrate the synthetic utility of this method.
Article information: https://doi.org/10.1038/s41467-025-63875-4
