Visible-Light-Induced Monofluoromethylation of Styrenes
Zhengling Wu1, Zechao Liu1, Junrui Wang1, Xuefeng Gu2, Huiming Dai2, Zhibin Huang1(黄志斌)*, Yingsheng Zhao1(赵应声)*
1Key Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou 215123, P. R. China
2Yonghua Chemical Co., Ltd., Changshu, 215500, P. R. China
Org. Lett. 2025, 27, 12368–12373
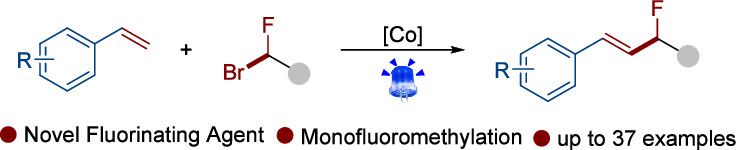
Abstract: The allylic monofluoromethyl moiety is a structurally significant motif with well-established relevance in pharmaceutical chemistry and materials science. This study presents a streamlined and effective method for the synthesis of allylic monofluoromethylated compounds. Utilizing sulfonyl monofluoromethyl bromide as the fluoromethylating agent, the transformation is facilitated under visible-light irradiation in the presence of a cobaloxime catalyst. This approach enables the direct fluoroalkylation of alkenes, affording the desired allylic monofluoromethyl products with high efficiency. The reaction proceeds under mild conditions and exhibits a broad substrate scope, displaying excellent functional group tolerance across various biologically important molecules, including derivatives of estrone, thymol, amino acids, glucose, and ciprofibrate.
Article information: https://doi.org/10.1021/acs.orglett.5c04012
