Synthesis and Reactivity of a Crystalline Zinc-cAAC Radical
Shuilian Xu1,2, Yizhen Chen3, Shengjie Jiang1,2,Gengwen Tan3(谭庚文)*, Xin Xu1,2(徐信)*
1Key Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou, 215123 P.R. China
2State Key Laboratory of Bioinspired Interfacial Materials Science, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou, 215123 P.R. China
3Key Laboratory of Bioinorganic and Synthetic Chemistry of Ministry of Education, Guangdong Basic Research Center of Excellence for Functional Molecular Engineering, School of Chemistry, IGCME, Sun Yat-sen University, Guangzhou, 510275 P. R. China
Angew. Chem. Int. Ed. 2025, 64, e202515723
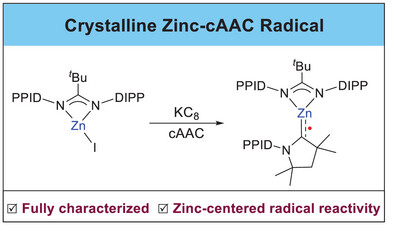
Abstract:Reaction of LAmZnI [LAm =tBuC(N-DIPP)2, DIPP = 2,6-iPr2-C6H3] with KC8 in the presence of cyclic (alkyl)(amino)carbene (cAAC) affords a stable radical complex [LAmZn(cAAC)]• (3). Single-crystal structural analysis of3 shows a short Zn─C bond and concomitant elongation of C-N bond within the cAAC ligand, indicating a significant π-backbonding from the metal to the cAAC ligand. EPR spectroscopy and DFT calculations reveal that the spin density is mainly localized on the carbenic carbon atom, with a small portion on the zinc center. Complex3 exhibits zinc-centered radical reactivity as demonstrated by reactions with 2, 2, 6, 6-tetramethyl-1-piperidinyloxy, iodobenzene, trimethylsilyl azide, and [FeCp(CO)2]2, all of which occur at the zinc center and yield the corresponding Zn(II) complexes.
Article information: https://doi.org/10.1002/anie.202515723
