Atmospheric Corrosion-Driven Redox Coordination Enables Spontaneous Carbonate Conversion of Lithium-Ion Battery Cathodes
Wendi Tang1, Tianran Yan2, Wenbin Dai1, Kaidan Shen1, Tingting Zhang1, Jialong Yu1, Liang Zhang2(张亮)*, Wei Zhang1(张伟)*
1State Key Laboratory of Bioinspired Interfacial Materials Science, Innovation Center for Chemical Science, College of Chemistry Chemical Engineering and Materials Science, Soochow University, Suzhou 215123, P. R. China
2Institute of Functional Nano & Soft Materials (FUNSOM), Soochow University, Suzhou 215123, P. R. China
J. Am. Chem. Soc.2025, 147, 35456–35463
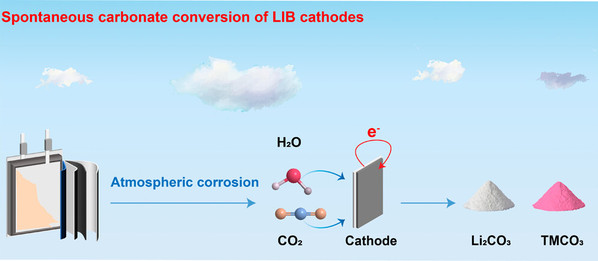
Abstract:The growing demand for lithium-ion batteries (LIBs) has intensified the need for sustainable methods to recover critical metals. Current recycling strategies, including pyrometallurgy and hydrometallurgy, often involve high energy consumption, harsh reagents, and poor selectivity, limiting their environmental and economic viability. Here, we report a chemically autonomous and energy-neutral cathode recycling process driven by atmospheric corrosion. Under humid air and dissolved CO2, aluminum current collectors undergo sustained oxidative dissolution, releasing electrons that selectively reduce transition metals (TM) in the layered oxide to TM(II). This redox reaction triggers H+ insertion, Li+/H+ exchange, and lattice destabilization, forming LixHyTMO2 intermediates that spontaneously coordinate with CO32– to yield TMCO3 or Ni2(OH)2CO3, while Li+ precipitates as Li2CO3. CO2 acts both as a proton carrier and as a coordinating ligand, sustaining the redox–coordination loop. This chemistry-driven approach redefines corrosion as a tool for selective redox transformations and offers a reagent-free, scalable pathway for LIB recycling under ambient conditions.
Article information: https://doi.org/10.1002/adfm.202516637