Key Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science, Soochow University
Advanced Synthesis & Catalysis,Volume 358, Issue 10, pages 1571–1576, May 19, 2016
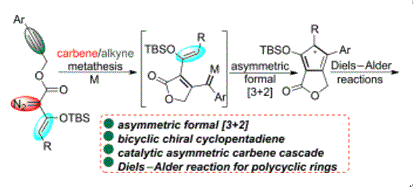
An asymmetric carbene cascade reaction, which proceeds through carbene/alkyne metathesis and formal (3 + 2) cycloaddition and converts alkynyl-tethered enol diazoacetates to chiral bicyclic cyclopentadienes, is presented; and no catalytic asymmetric version of these carbene cascade transformations has been disclosed so far. The proton signals of the cyclopropene intermediates are observed in the mechanism study, which clearly demonstrate the reaction pathways. In addition, these products are intercepted via Diels-Alder reactions to provide bridged polycyclic structures in high yields and enantioselectivities.
链接:http://xueshu.baidu.com/s?wd=paperuri%3A%2827ed7d8bd223f51f4c0817424c529b3b%29&filter=sc_long_sign&tn=SE_xueshusource_2kduw22v&sc_vurl=http%3A%2F%2Fonlinelibrary.wiley.com%2Fdoi%2F10.1002%2Fadsc.201501106%2Fcitedby&ie=utf-8&sc_us=7290717290696655441