A benzoxazine–hemicyanine based probe for the colorimetric and ratiometric detection of biothiols
Xiao-Dong Liua, Ru Suna*(孙如), Ying Xub, Yu-Jie Xub, Jian-Feng Gea*(葛健锋), Jian-Mei Lua, c*(路建美)
a Key Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Material Science, Soochow University, Suzhou 215123, China
b School of Radiation Medicine and Protection, Medicine College of Soochow University, Suzhou 215123, China
c Key Laboratory of Functional Materials for Environment Protection of Suzhou City, Soochow University, Suzhou 215123, China
Sensor. Actuat. B-Chem. 2013, 178, 525–531
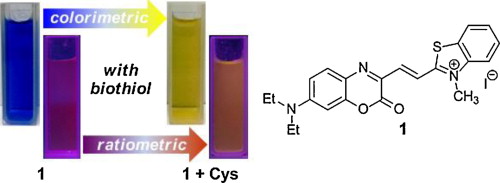
Aliphatic biothiols, including cysteine, homocysteine, and glutathione, are associated with a wide range of biological functions. Hence, the selective detection of intracellular thiols has significance for investigating cellular function. Ratiometric fluorescent probes are very useful in the biological sciences because they allow measurement at two emission wavelengths, providing a built-in correction for environmental effects. In this study, a benzoxazine–hemicyanine based probe (1) was synthesized and characterized. The probe shows quick colorimetric and ratiometric response toward biothiols in the presence of other biomolecules and it functions in a wide pH range exhibiting large shifts in both absorption and fluorescence spectra. The detection mechanism, which was induced by the addition of mercaptan toward the activated double bond in the probe, was verified using 1H NMR and mass spectrometry analysis. The photophysical properties changes that occurred upon the reaction of the probe were found to be induced by the variation of intermolecular charge transfer, which was confirmed by theoretical calculations based on density functional theory (DFT). The fluorescence imaging of intracellular thiols was also examined with excellent performance.
链接: http://www.sciencedirect.com/science/article/pii/S0925400512014281