Pressure-Assisted Fast Synthesis of Zeolitic Materials
Kai Zhang1, Guangyuan He2,3, Ning Wang4, Zhuoya Dong5, Yanhang Ma5, Jun Xu6, Donghai Mei2,3, Qiming Sun1(孙启明)*, Jihong Yu7(于吉红)*
1Innovation Center for Chemical Science, College of Chemistry, Chemical Engineering and Materials Science, Jiangsu Key Laboratory of Advanced Negative Carbon Technologies, Soochow University, Suzhou 215123, P. R. China
2School of Materials Science and Engineering and School of Environmental Science and Engineering, Tiangong University, Tianjin 300387, P. R. China
3School of Environmental Science and Engineering, Tiangong University, Tianjin 300387, P. R. China
4Institute of Sustainable Energy and Resources, College of Chemistry and Chemical Engineering, Qingdao University, Qingdao 266071, P. R. China
5School of Physical Science and Technology, ShanghaiTech University, Shanghai 201210, P. R. China
6National Center for Magnetic Resonance in Wuhan, State Key Laboratory of Magnetic Resonance Spectroscopy and Imaging, Wuhan Institute of Physics and Mathematics, Innovation Academy for Precision Measurement Science and Technology, Chinese Academy of Sciences, Wuhan 430071, P. R. China
7State Key Laboratory of Inorganic Synthesis and Preparative Chemistry, College of Chemistry, Jilin University, Changchun 130012, P. R. China
J. Am. Chem. Soc., 2025, 147, 26277–26285
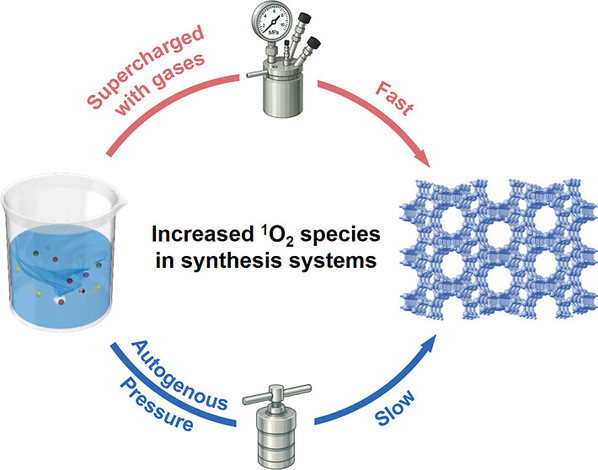
Abstract:Zeolites are widely utilized in various industrial applications, such as ion exchangers, catalysts, and adsorbents. However, traditional zeolite crystallization is time-intensive, requiring hours to weeks under autogenous pressure in autoclaves. In this study, we developed a novel and universal pressure-assisted method for the rapid synthesis of diverse zeolites, including MFI, CHA, FAU, MOR, *BEA, LTA, and AFI, as well as zeolite-encaged ultrasmall metal clusters and atoms, using batch reactors supercharged with controlled amounts of gases. Compared to traditional hydrothermal synthesis, this method significantly accelerates the crystallization rate of zeolites by 2–18 times. Experimental and theoretical analyses reveal that an elevated synthesis pressure and increased oxygen content in the system promote the formation of singlet oxygen species, thereby facilitating T–O–T (T═Si, Al, and P) bond restructuring and accelerating zeolite nucleation. This work offers a practical and efficient pathway for rapidly synthesizing zeolites to meet industrial demands while shedding light on the underlying mechanisms of zeolite crystallization.
Article information: https://doi.org/10.1021/jacs.5c04247