Zinc substituted carbenes: synthesis, structure, and ambiphilic reactivity
Shengjie Jiang1(蒋胜杰), Ganping Wang2, Yanping Cai1, Laurent Maron2*, Xin Xu1(徐信)*
1Key Laboratory of Organic Synthesis of Jiangsu Province, State Key Laboratory of Bioinspired Interfacial Materials Science, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou 215123, P. R. China
2LPCNO, CNRS, INSA, Université Paul Sabatier, 135 Avenue de Rangueil, 31077 Toulouse, France
Chem. Sci.,2025, 16, 15462-15468
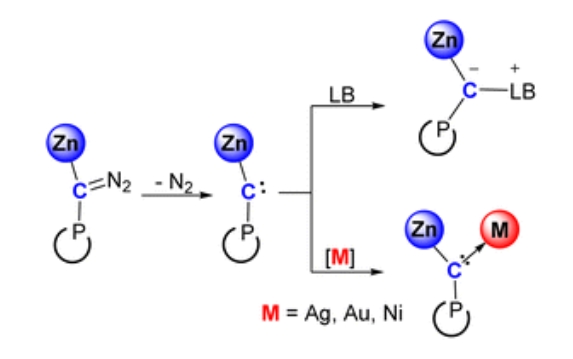
Abstract: Metal-substituted carbenes are fundamentally important as they represent the limiting configurations of metal carbynes. However, structurally characterized examples are still rare, and their reactivity remains underexplored. Herein, we report the first synthesis, characterization, and reactivity studies of zinc-substituted carbenes. UV irradiation of zinc diazoalkyl complexesLZnC(N2)P [L = [(ArNCMe)2CH]−,P= (DippNCH2)2P, Ar = Dipp or Mes, Dipp = 2,6-iPr2C6H3, Mes = 2,4,6-Me3C6H2] generates Zn(II)-substituted carbenesLZnCP with concomitant N2 release. The Zn–C–P moiety features nearly linear carbene centers, deviating from conventional carbene geometry. Computational studies indicate a singlet ground state stabilized through synergistic effects of C–P π-interaction and carbene lone-pair delocalization towards the Zn center. Treatment ofLZnCP with CO2 selectively affords zincated ketene via nucleophilic attack and tandem C[double bond, length as m-dash]O double bond cleavage. It reacts with 4-dimethylaminopyridine to form a carbene-Lewis base adduct exhibiting electrophilic reactivity. Furthermore, zinc-substituted carbenes enable direct transition metals coordination to give the heterobimetallic Zn/M (M = Ag+, Au+, Ni) μ-carbyne complexes.
Article information: https://doi.org/10.1039/D5SC03342J
