Palladium-Catalyzed "Cut-and-Sew" Reaction of C(sp3)-NC: Direct Synthesis of Bulky Nitriles Containing an All-Carbon Quaternary Center
Ming Yang1,2, Yi-Ming Zhu1,2, You Zi3(訾由)*, Shun-Jun Ji1,2(纪顺俊)*, Xiao-Ping Xu1,2,4(徐小平)*
1Key Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou 215123, P. R. China
2Collaborative Innovation Center of Suzhou Nano Science and Technology, Soochow University, Suzhou 215123, P. R. China
3School of Chemistry and Chemical Engineering, Nantong University, Nantong 226019, P. R. China
4Innovation Center for Chemical Science, Soochow University, Suzhou 215123, P. R. China
Org. Lett. 2025, 27, 6755–6760
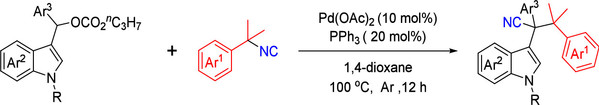
Abstract: A palladium-catalyzed carbo-cyanation reaction for synthesizing bulky nitriles with an all-carbon quaternary center via a “cut-and-sew” process of isocyanides is presented. The reaction proceeds through oxidative addition of indole-derived carbonate to Pd(0), followed by decarboxylation, migratory insertion, and the formation of a ketenimine intermediate. A geminate radical pair is formed, leading to radical coupling and the final bulky nitrile product. The method demonstrates high efficiency and a wide substrate tolerance, offering a valuable strategy for constructing bulky nitriles in organic synthesis with potential applications in drug development.
Article information: https://doi.org/10.1021/acs.orglett.5c01914
