Cobalt-Catalyzed Alkene Alkylarylation: Access to Pyrrolidinones
Junda Wang1, Kaixin Chen1, Changrui Nie1, Chenhao Huang2,3, Qian Zhang2,3(张倩)*, Jie Li4(李杰)*
1Key Laboratory of Organic Synthesis of Jiangsu Province, Suzhou Key Laboratory of Pathogen Bioscience and Anti-infective Medicine, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou 215123, China;
2Department of Chemistry and Materials Science, Advanced Materials Research Center, School of Science, Xi’an-Jiaotong Liverpool University, Suzhou, Jiangsu 215123, China;
3Department of Chemistry, University of Liverpool, Liverpool L69 7ZD, U.K;
4State Key Laboratory of Bioinspired Interfacial Materials Science, MOE Key Laboratory of Geriatric Diseases and Immunology, Key Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou 215123, China
Org. Lett.2025, 27, 9287
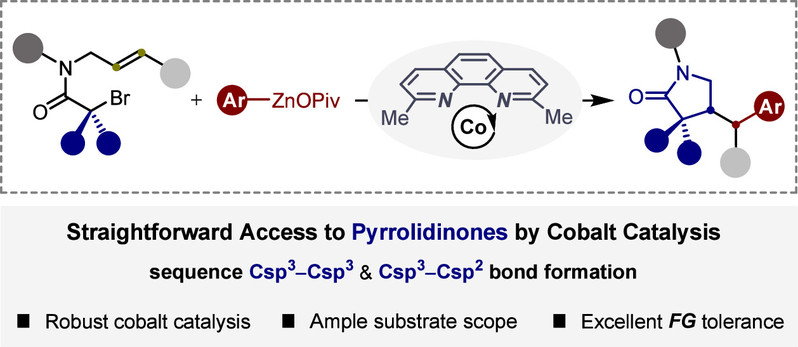
Abstract:Practical cyclization and cross-coupling reactions between alkenyl-substituted alkyl bromides and arylzinc pivalates via cobalt-catalyzed sequential C(sp3)-C(sp3)/C(sp3)-C(sp2) bond formation have been disclosed, thus providing straightforward access to structurally diverse pyrrolidinones. Moreover, this approach could be also effective for the modular preparation of spirocyclic pyrrolidinones through spirocyclized alkene alkylarylation. Beyond that, rather mild conditions, synthetic simplicity, and excellent functional group compatibility show the potential applications of this method in synthetic chemistry.
Article information: https://doi.org/10.1021/acs.orglett.5c02870
