, , 汪顺义), and 纪顺俊)
Key Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science and Collaborative Innovation Center of Suzhou Nano Science and Technology, Soochow University, Suzhou215123, China
Org. Lett., 2018, 20 (19), 6112--6116
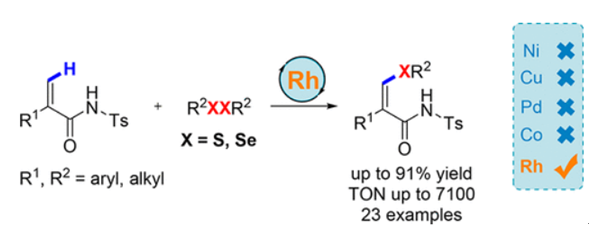
A regioselective rhodium-catalyzed thiolation of N-tosyl acrylamides with readily available disulfides has been developed. Through N-tosylamide-assisted activation of the alkenyl C(sp2)–H bond, a series of (Z)-alkenyl sulfides were constructed in moderate to excellent yields with good tolerance of functional groups. Turnover numbers (TONs) of up to 7100 were obtained utilizing 0.01 mol % RhIII catalyst. In addition, diphenyl diselenide was also successfully applied to this reaction for the construction of (Z)-β-alkenyl selenides under identical conditions.
链接:https://pubs.acs.org/doi/10.1021/acs.orglett.8b02552