a Key Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou 215123, P. R. China
b College of Physics, Optoelectronics and Energy & Collaborative Innovation Center of Suzhou Nano Science and Technology, Soochow University, Suzhou 215006, P. R. China
Chem. Commun., 2016,52, 10241-10244
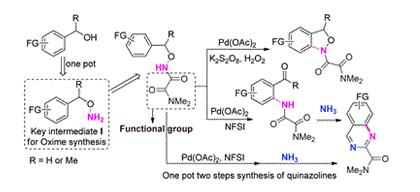
Direct transformation of a directing group to important synthetic units would provide a high atom efficiency synthetic approach in synthetic chemistry. Herein, a convenient protocol for the synthesis of o-aminobenzaldehyde and benzoxazole derivatives from benzyl alcohols has been developed by employing (N,N-dimethyl)oxamoyl amide as a directing group in a palladium-catalyzed intramolecular amination. Furthermore, the attached directing center may not only be transformed into the product, but may also be further applied to generate synthetically important quinazoline and quinoline units. Finally, a high atom efficiency one-pot, two-step approach to form quinazolines from benzyl alcohol derivatives has been achieved in good yields, thus demonstrating its high utility.
链接:http://pubs.rsc.org/EN/content/articlelanding/2016/cc/c6cc05560e#!divAbstract