Welcome to College of Chemistry, Chemical Engineering and Materials Science of Soochow
University
A Bimetallic Fe−Mn Oxide-Activated Oxone for In Situ Chemical Oxidation (ISCO) of Trichloroethylene in Groundwater: Efficiency, Sustained Activity, and Mechanism Investigation
Xueying Yang1, Jingsheng Cai2, Xiaoning Wang1, Yifan Li3, Zhangxiong Wu1, Winston Duo Wu1, Xiao Dong Chen1, Jingyu Sun2, Sheng-Peng Sun1*(孙胜鹏), and Zhaohui Wang4
1 School of Chemical and Environmental Engineering, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou, Jiangsu 215123, China
2College of Energy, Soochow Institute for Energy and Materials Innovations (SIEMIS), Soochow University, Suzhou, Jiangsu 215006, China
3 Key Laboratory for Yellow River and Huai River Water Environmental and Pollution Control, Ministry of Education, Henan Key Laboratory for Environmental Pollution Control, School of Environment, Henan Normal University, Xinxiang, Henan 453007, China
4 Shanghai Key Lab for Urban Ecological Processes and Eco-Restoration, School of Ecological and Environmental Sciences, East China Normal University, Shanghai 200241, China; Institute of Eco-Chongming (IEC), Shanghai 200062, China
Environ. Sci. Technol.2020,54, 3714--3724
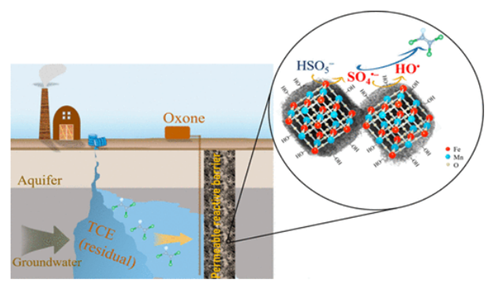
Bimetallic Fe–Mn oxide (BFMO) has been regarded as a promising activator of peroxysulfate (PS), the sustained activity and durability of BFMO for long-term activation of PS in situ, however, is unclear for groundwater remediation. A BFMO (i.e., Mn1.5FeO6.35) was prepared and explored for PS-based in situ chemical oxidation (ISCO) of trichloroethylene (TCE) in sand columns with simulated/actual groundwater (SGW/AGW). The sustained activity of BFMO, oxidant utilization efficiency, and postreaction characterization were particularly investigated. Electron spin resonance (ESR) and radical scavenging tests implied that sulfate radicals (SO4•–) and hydroxyl radicals (HO•) played major roles in degrading TCE, whereas singlet oxygen (1O2) contributed less to TCE degradation by BFMO-activated Oxone. Fast degradation and almost complete dechlorination of TCE in AGW were obtained, with reaction stoichiometry efficiencies (RSE) of ΔTCE/ΔOxone at 3–5%, much higher than those reported RSE values in H2O2-based ISCO (≤0.28%). HCO3– did not show detrimental effect on TCE degradation, and effects of natural organic matters (NOM) were negligible at high Oxone dosage. Postreaction characterizations displayed that the BFMO was remarkably stable with sustained activity for Oxone activation after 115 days of continuous-flow test, which therefore can be promising catalyst for Oxone-based ISCO for TCE-contaminated groundwater remediation.
链接:https://pubs.acs.org/doi/abs/10.1021/acs.est.0c00151