Welcome to College of Chemistry, Chemical Engineering and Materials Science of Soochow
University
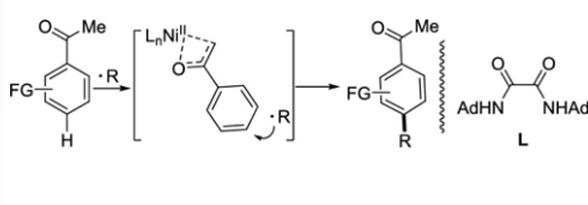
Nickel-Catalyzed, para-Selective, Radical-Based Alkylation of Aromatic Ketones
Jie Wang†, Yu-Bo Pang†, Na Tao, Runsheng Zeng*(曾润生), and Yingsheng Zhao*(赵应声)
College of Chemistry Chemical Engineering and Materials Science,Soochow University, Suzhou, China
† J.W. and Y.P. contributed equally.
Org. Lett.2020, 22, 854--857
A direct, para-selective, radical-based alkylation of aromatic ketones with alkanes has been developed using a nickel catalyst with oxamide as the ligand. Acetophenones bearing electron-withdrawing substituents were functionalized directly with simple alkanes with high para-selectivity while acetophenones with electron-donating groups were mainly para-functionalized. A mechanistic study indicated that C–H bond activation of the aromatic ring may be the rate-determining step of the reaction.
链接:https://pubs.acs.org/doi/abs/10.1021/acs.orglett.9b04327