Welcome to College of Chemistry, Chemical Engineering and Materials Science of Soochow
University
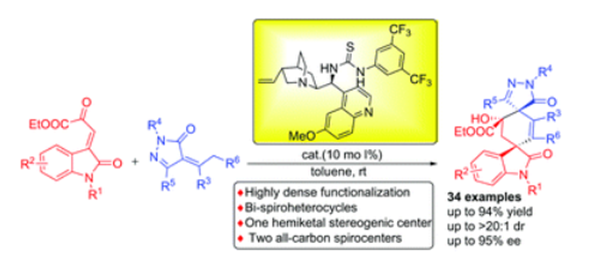
Organo-catalyzed asymmetric cascade annulations reaction for the construction of bi-spirocyclic pyrazolone and oxindole derivatives
Bing-Bing Sun a, Jun-Bo Chen a, Jun-Qi Zhang a, Xiao-Peng Yang a, Hao-Peng Lv a, Zheng Wang*b(王正)and Xing-Wang Wang *a(王兴旺)
aKey Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science Soochow University, Suzhou 215123, China
bState Key Laboratory of Organometallic Chemistry, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, Shanghai 200032, China
Org. Chem. Front., 2020, 7, 796--809
A bifunctional organocatalyst catalyzed tandem annulation reaction between β,γ-unsaturated α-ketoesters with α-arylidene pyrazolinones was reported. The tandem Michael–aldol process allows access to optically active bi-spirocyclic pyrazolone and oxindole compounds in high yields with good to excellent enantioselectivity and diastereoselectivity. The resulting bi-spirocyclic compounds possess highly dense functionalized substituents, two all-carbon spiro quaternary stereocenters and one hemiketal stereogenic centers.
链接:https://pubs.rsc.org/en/content/articlelanding/2020/qo/d0qo00001a#!divAbstract