Welcome to College of Chemistry, Chemical Engineering and Materials Science of Soochow
University
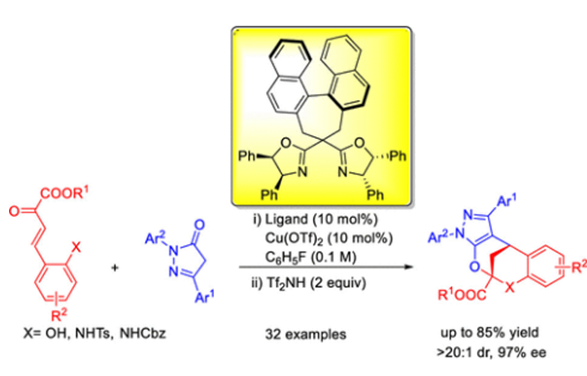
Chiral Binaphthyl Box-Copper-Catalyzed Enantioselective Tandem Michael−Ketalization Annulations for Optically Active Aryl and Heteroaryl Fused Bicyclicnonanes
Wei-Tai Fan1, Xiao-Peng Yang1, Hao-Peng Lv1, Xing-Wang Wang1*(王兴旺), and Zheng Wang2*(王正)
1Key Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou 215123, the People’s Republic of China
2State Key Laboratory of Organometallic Chemistry, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, Shanghai 200032, the People’s Republic of China
Org. Lett. 2020, 22, 3936--3941
The combination of chiral binaphthyl box-copper(II) with triflimide (Tf2NH) was identified as an efficient catalytic system for the asymmetric Michael/ketalization of (E)-2-hydroxyaryl-2-oxobut-3-enoates or (E)-ethyl 4-(2-aminoaryl)-2-oxobut-3-enoates with pyrazolone derivatives. The corresponding asymmetric tandem reactions provided a series of enantioenriched aryl and heteroaryl fused 2,8-O,O- or O,N-bicyclo[3.3.1]nonanes in high yields with excellent enantio- and diastereoselectivities.
链接:https://pubs.acs.org/doi/abs/10.1021/acs.orglett.0c01221